Multimedia Site Education and Centralized Tracking
DrugDev’s Learning Management System (LMS)offers a single, comprehensive repositoryfor sites and sponsors providing multimedia education, compliance reporting, knowledge assessments, completion certificates and expiration tracking.
Sponsor benefits…
- Bulk distribution of training with customized, role-based distribution lists
- Comprehensive and detailed tracking reports
- Drill-down detail of site assessment answers to identify areas for additional education
- Track and generate certificates for online modules, IM, on-site meetings, site initiation visits and more
- Automatically identify and remind sites who are past due
- Fully validated and compliant with 21 CFR Part 11 requirements
Site benefits…
- User-based curriculum
- Video and audio modules
- Interactive assessments
- Automatic due-date reminders
- Deliver sponsor specific and study specific training materials
- Single-sign on for sites across multiple studies and sponsors
- Periodically saves progress to allow users to resume training when interrupted or signing off
- Integrated support documents and guides
How it Works
Step One:
Dynamic distribution lists allow bulk training assignments to be pushed out on demand, or through a pre-determined schedule. Then, filters allow training assignment by geographic location and trial role. In addition, training modules are automatically pushed to new users and hires based on their role.
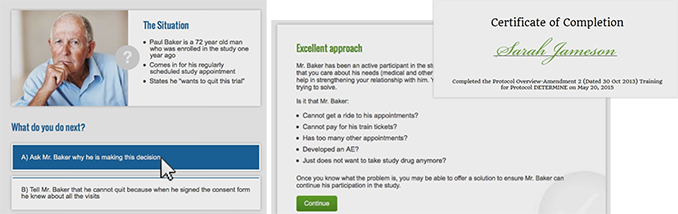
Step Two:
Sites receive on demand access to multimedia training modules. These modules are interactive with assessments that tests sites according to your study. The system logs the progress of the training, allowing sites to pick up where they left off. It also provides an electronic certificate of completion as proof for sponsors.
Step Three:
Sponsors can view the assessment scores of sites while viewing their responses to individual questions. They also receive automatic notification of past-due sites with the ability to set and send automated training reminders.